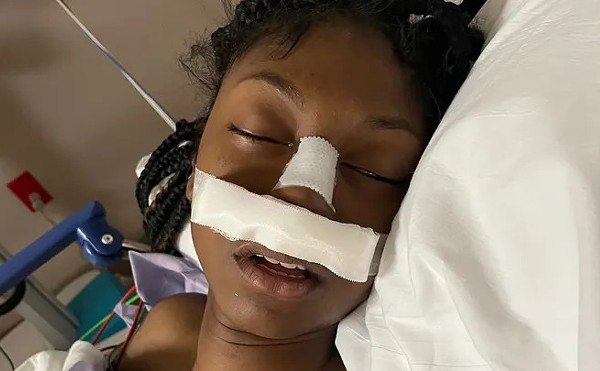
Robert Stanley calls his son Payton "a multi-million-dollar man." Before Payton was even born he had a stroke in his mother's womb during the third trimester. At two months old, he began having seizures, at times more than 100 in a day. He's had a portion of his brain removed. Today, at age five, he requires a specialized breathing treatment and cannot walk. The family makes regular trips from their home in Vinita, Oklahoma, to Memphis' LeBonheur Children's Hospital, which is widely recognized for its pediatric neurology and epileptology care. Stanley has worked two jobs so that Payton's mother Stephanie can stay home full time. Payton requires 30 medications every day, many of which cost, in Stanley's words, an "extremely stupid" amount of money.
I called Stanley last month to talk about the cost of one medicine in particular. Acthar is an injectable drug manufactured by St. Louis-based Mallinckrodt Pharmaceuticals and used to treat infantile spasms. Payton was on it for eight months in 2015. Disappointed with the pediatric care they found in Oklahoma, where doctors had misdiagnosed Payton, the family had made their first trip to LeBonheur. The doctors there diagnosed Payton with infantile spasms and prescribed Acthar. And despite the litany of high-cost medications that have been prescribed to Payton over the years, the sticker shock that came with Acthar still sticks in Stanley's memory.
"I heard about the price when I went to go pick up the medicine. I asked the representative that night, 'How much is this stuff?'"
The representative's answer: "Fifty-thousand dollars a vial."
"I about had a heart attack," Stanley told me.
A standard Acthar treatment lasts a month and requires two to three vials. Payton ended up being on the drug for eight weeks.
Fortunately, the family's insurance covered the treatment, though it ended up being ineffective for Payton, who had to eventually have a portion of his brain removed in order to treat the seizures. At least they hadn't been bankrupted by Acthar.
However, someone had to pay for those $50,000 vials. And about a year after Stanley first heard of Acthar, it also came to the attention of Larry Morrissey, then the mayor Rockford, Illinois. Like Stanley, Morrissey couldn't believe the price. Unlike Stanley, Morrissey's city actually had to pay.
Rockford is a city of about 150,000 people, 90 miles west of Chicago. Like a lot of Midwestern cities, it has suffered from crime, depopulation and an image problem, occasionally ranking high on those dubiously researched "Worst Places to Live" articles. Morrissey, a Democrat, was elected on an agenda of cost saving and infrastructure improvements. One of those cost-saving initiatives was to find out exactly how much the city, which funds its own health plan for municipal workers, was spending on health care. In 2016, the auditors noticed something unusual: The previous year, the city had spent nearly half a million dollars on a drug called Acthar. Only two people on the city's health plan took the injectable drug, and between them they took a total of just nine vials. That came out to more than $54,000 a vial.
"These are funds that could have been directed to public infrastructure, economic development or crime reduction," Rockford spokesperson Laura Maher says. "[Half a million] is equivalent to approximately five police officers annually."
In the year 2000, a vial of Acthar sold for just $40. The two-decades-long story of how this drug saw its price increase from $40 to $54,000 is riddled with allegations of kickbacks, sketchy marketing and a "murky alliance" between a drug manufacturer and America's biggest pharmacy benefit manager. Key to the story are two of St. Louis' biggest corporations.
In 2017, Rockford filed a lawsuit against the drug's manufacturer, St. Louis-based Mallinckrodt, and the drug's sole distributor, Express Scripts, which also has its headquarters in St. Louis. Rockford's suit accuses the two companies of colluding to artificially raise the price of the drug. It also accuses Mallinckrodt of essentially paying doctors to prescribe Acthar even when cheaper, more suitable drugs were available.
The RFT reached out to Mallinckrodt and Express Scripts for comment. Representatives of both entities replied that they cannot comment on pending litigation.
U.S. Magistrate Judge Iain D. Johnston, who is handling the early portion of the Rockford case, wrote in a pre-trial opinion, "The issues at stake in these cases are large ... [T]he potential amount in controversy is extraordinary. The cases contain racketeering and antitrust claims, which, if successful, could lead to huge damage awards. In today's legal vernacular, these are 'bet the company' cases."
From a pig's gland to a $6 billion company
Acthar is the brand name for the naturally occurring adrenocorticotropic hormone (ACTH). In the 1940s, physician Philip Hench and chemist Edward Kendall discovered it could be extracted from the pituitary gland of a pig (usually after the rest of the pig had been butchered for meat) and injected into a human. ACTH causes humans to create more cortisone, which reduces inflammation, and for decades Hench and Kendall's discovery worked wonders for people who suffered from rheumatoid arthritis. The two men later won a Nobel Prize in Physiology or Medicine for their work.
But as decades passed, advances in medicine led to other anti-inflammatory therapies, such as Prednisone, which can be manufactured cheaply and don't come with the inevitable trace impurities that are extracted from a pig's gland along with ACTH.
By the 1990s, ACTH was no longer the preferred treatment for anything except infantile spasms, or IS, a form of epilepsy that is as rare as it is serious.
Pediatric neurologist Jim Wheless told me that there are about 2,000 new cases of IS a year. "If these kids are not treated timely with effective medication, then almost all of them will go on to have significant mental impairment," he said.
Wheless added that when it comes to treating IS, there are really only two FDA-approved medications, and of those two Acthar has been clearly shown to be superior, with an efficacy rate of about 90 percent.
"If it was your child or your grandchild and I'm sitting across the table saying, 'Gosh, if we don't jump on this and treat this effectively, the child is probably going to have significant mental challenges the rest of their life. There's only two medicines, and this one works better — what do you want to do?'" Wheless said. "You can guess what most families are going to opt for."
Wheless added, "Now, what if I tell you that the ideal medication is so expensive you have to mortgage your house? Or that you can't have a house?"
But a single vial of Acthar didn't always cost more than a down payment on a starter home.
In the 1990s, Acthar was a product of Aventis Pharmaceuticals, the only company producing ACTH of any kind. At that time, a vial of Acthar from Aventis cost about $40, and the low incidence of infantile spasms combined with the low price meant that Aventis lost money on the drug. They tried to stop producing it in the 1990s, but pediatricians intervened, saying that without Acthar parents whose children suffered infantile spasms would be without an important treatment.
In 2001, Aventis sold Acthar to California-based Questcor for just $100,000, making Questcor the only source for the first-line drug against infantile spasms. The company used this monopoly to essentially set its own price. Right after acquisition, Questcor raised the price of Acthar from $40 a vial to close to $800 a vial. The price grew steadily from there. By 2007, insurance companies and Medicare were paying nearly $2,000 a vial.
But that was just the beginning. In 2007, Don Bailey became CEO of Questcor. Over the next thirteen years, the "one-drug company" turned that one drug into a $6 billion business.
During the first year of Bailey's leadership, Questcor struck a deal with Express Scripts, making the St. Louis-based pharmacy benefits manager the sole distributor of Acthar.
Over 80 million people get their medications through Express Scripts, a buying volume that gives the company considerable power to negotiate lower prices from drug companies. The company regularly posts revenues of more than $100 billion a year.
However, Rockford's suit alleges, because of the exclusive distribution deal Express Scripts inked with Questcor, the e-pharmacy had no incentive to negotiate for a lower price and therefore did not do so.
In the months following the Questcor deal with Express Scripts, the price of Acthar went from $2,000 a vial to more than $29,000 a vial.
Eric Liebler was a senior vice president at Questcor around the time of the Express Scripts deal. Though he wasn't involved in the deal with the e-pharmacy, he was privy to the company's conversations about dramatically increasing the price of Acthar. He told me that prior to the deal the company drafted two plans for how it could increase the drug's worth.
Option one was to do a lot of research on Acthar, find new uses for it and, while doing so, raise the price gradually over several years in a way commensurate with the increased number of uses.
"Option two was, 'Let's just do it all at once,'" Liebler said. "You're going to get yelled at for raising the price no matter what you do, so go from $1,500 right up to $21,000."
Liebler says he recommended the first approach, doing it gradually. The company went with the second, dramatically raising the price in short order.
"I helped them write the press release, but then I resigned immediately," Liebler said. "There are many companies who have played the price-increase game, but I'd never seen anything like this at this magnitude."
By 2012, insurance companies were increasingly dubious about both the price of Acthar and its efficacy. Health insurer Aetna reduced reimbursements for Acthar, a spokeswoman telling Reuters at the time, "The decision was based on the lack of clinical evidence that the drug is more effective than existing steroids."
In the wake of Aetna's announcement, Questcor's stock price dropped by 40 percent.
However, good news for Questcor and their shareholders came in 2014 when St. Louis-based Mallinckrodt Pharmaceuticals paid Questcor $5.9 billion as part of a merger between the two companies. The merger raised eyebrows. At the time, Questcor was the subject of two federal whistleblower complaints, a Securities and Exchange Commission investigation related to the marketing of Acthar and an ominous 2012 New York Times article that highlighted the extreme price increase. "I have a Cadillac in my refrigerator," says one Acthar patient quoted in that article, referring to an unused 5 ml vial.
One month after the merger, Bailey, the Questcor CEO whose tenure coincided with most of Acthar's price increase, sold almost a quarter million shares of Mallinckrodt, worth nearly $19 million, according to stock market tracking tool Wallmine. Bailey also joined Mallinckrodt's board of directors.
"Most of the Questcor people who stayed when I was uncomfortable, they became very rich, ungodly amounts of money," Liebler said. "Their [stock] options were back at 40 cents, 80 cents, and they probably sold at one hundred bucks. When I write the checks for my daughter's college, I know that would have been easier financially to stay. But I did the right thing."
The controversy and litigation stemming from Acthar, its pricing and marketing have only increased since Mallinckrodt's $6 billion acquisition.
While the data supporting Acthar as an effective treatment for infantile spasms is strong, the market for IS treatments is limited given the rarity of the disease. Wheless, the pediatric neurologist, said that he also believes given Acthar's price it's only a matter of time until alternative, cheaper treatments for IS appear on the market.
Seeking to expand the market for Acthar, Questcor and Mallinckrodt have both promoted it as a treatment for conditions other than infantile spasms, and at times these promotions have drawn accusations that the drug is being promoted in ways outside its FDA approval.
In 2016, a man named Barry Franks who had worked in sales for Questcor and later Mallinckrodt filed a lawsuit against the company claiming that he was fired for not selling Acthar to doctors based on uses that were not FDA approved. Acthar is FDA approved as an additional treatment for acute flare-ups of rheumatoid arthritis, or RA, but Franks claimed salespeople were incentivized to promote the drug and encourage refills as a long-term therapy to manage RA. Express Script's own prior authorization process stated that in treating rheumatic disorders, Acthar is only approved for "short term administration for an acute episode."
Furthermore, Franks' suit claimed the salaries for Mallinckrodt's sales staff were pegged directly to their ability to push Acthar to doctors as a long-term RA treatment. In addition to base pay, salespeople took part in an "Incentive Bonus Plan" — according to Franks this was referred to within the company as the "Rheum Incentive Plan" — through which salespeople could potentially earn more than their base salary.
Meanwhile, by 2018, four years after Mallinckrodt acquired Acthar, the price of one vial reached $40,000.
In addition to what Acthar is promoted as a treatment for, there are also considerable concerns about who is doing the promotion.
Spokes-doctors
Around the same time as the 2007 Express Scripts deal, Rockford's lawsuit claims, Questcor began paying doctors to aggressively market Acthar to other doctors who were then financially compensated for prescribing Acthar to their patients. These spokespeople/experts were called Key Opinion Leaders (KOLs), and Rockford's lawsuit — as well as a suit brought by the Department of Justice — alleges that the money from Questcor motivated them to promote Acthar in ways unsubstantiated by empirical research. The Rockford lawsuit, data from a ProPublica investigation and an article published in the Journal of American Medicine all indicate that Mallinckrodt continued this practice after merging with Questcor.
One of these KOLs was Maryland neurologist Ruwani Gunawardane, who according to government data in 2013 and 2014 received almost $150,000 from Questcor. From 2015 to 2018, she received $199,000 from Mallinckrodt in honoraria and for speaking, training and education.
A 2018 lawsuit brought by the Pennsylvania-based International Union of Operating Engineers Local 542 claimed, like Rockford's suit, that their health plan paid an inflated price for Acthar. The IUOE suit also alleges that Gunawardane's promotion of the drug to other doctors relied heavily on Acthar's off-label uses.
According to the NIH, "The FDA prohibits the promotion of drugs and devices for off-label uses by companies."
"These companies, and Mallinckrodt especially, have figured out that if we get a doctor to make that pitch, they think they're not marketing in violation of the law," said Don Haviland, the attorney representing Rockford. "That's why they pay big money to these doctors to go out and hawk for them."
According to the IUOE suit, Gunawardane trained other doctors to become KOLs on Acthar, including doctors who went on to prescribe Acthar to patients whose insurance was through the labor union.
Irene Greenhouse, a neurologist practicing in a suburb north of Philadelphia, is one of the doctors who the IUOE suit alleges prescribed Acthar not on the basis of it being the best treatment, but because she received money from Mallinckrodt. The suit alleges that Greenhouse prescribed one of the IUOE's health plan members Acthar for multiple sclerosis (due to HIPAA protections the patient's name is unknown). The suit claims Greenhouse prescribed Acthar in lieu of drugs that would have been cheaper and more appropriate, especially given that the patient was trying to get pregnant. The warning on Acthar's label states, "H.P.Acthar should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus."
Furthermore, Express Scripts' own prior authorization process stated, "Regarding MS, there is no evidence that Acthar impacts the ultimate outcome or natural history of the disease."
The FDA has approved Acthar for MS flare-ups, but not for management of the chronic disease.
The IUOE suit also alleges Greenhouse went so far as to submit false information about the patient's medication history, claiming that other medications the patient had not taken had failed to treat her MS. In 2016, Greenhouse received $37,000 in compensation from Mallinckrodt, according to ProPublica.
"When a doctor prescribes a medication like this, why isn't it part of their Hippocratic Oath to say, 'Oh and by the way, just so you know, this company gave me $37,000 last year'?" Haviland said.
In 2012, two Questcor employees filed whistleblower complaints in federal court detailing the system of kickbacks and other compensation that Questcor (as well as Mallinckrodt after merging with Questcor) used to boost Acthar prescriptions. The whistleblower complaints, which were unsealed in 2019, were brought by Charles Strunck and Lisa Pratta, both of whom worked in sales for Questcor. Pratta also worked for Mallinckrodt after the merger.
Strunck and Pratta claimed that Questcor "cheated the federal government [Medicare] out of millions of dollars" through systematic kickbacks to health care providers in exchange for them promoting and prescribing Acthar.
The whistleblower complaint also claims that Questcor gave large sums of money to the nonprofit Chronic Disease Fund, which reimbursed Acthar users for their copays. On the surface, helping users pay for their medication might seem noble, but the whistleblower suit described how doing so enabled Questcor to "cheat" the taxpayers out of millions.
Americans on Medicare often pay out of pocket for a percentage of their drug costs. If patients had to pay even 5 percent of the $40,000 price for a single vial of Acthar, many would have no choice but to go without. The whistleblower suit alleged that Questcor and later Mallinckrodt used the Chronic Disease Fund to subsidize the patients' copays so they wouldn't complain about the high costs, leaving Medicare and insurance companies stuck footing the remaining tens of thousands of dollars. It's legal for a drug company to subsidize patients who have private insurance, but illegal to do so for patients on Medicare.
A ProPublica investigation found that Acthar "isn't prescribed often, just 3,387 times in Medicare in 2012. But Part D spent an average of $41,763 per prescription, making it one of the most expensive drugs around." According to government data, Medicare Part D spent $724 million on the drug in 2018.
As Acthar became prescribed more liberally, the reports of adverse side effects associated with its use increased as well. In 2017, Citron Research analyzed data from the FDA's Adverse Reporting System and found that in 2016, two years after Mallinckrodt acquired Acthar, 82 people died due to complications associated with the medication. This was up from only four deaths in 2012.
Mallinckrodt paid $100 million in a 2017 settlement to resolve Federal Trade Commission charges that the company maintained a monopoly over Acthar.
In June 2019, the Department of Justice joined the Strunck and Pratta whistleblower suits. Mallinckrodt responded to the allegations by claiming in a statement they "pertained principally to legacy Questcor conduct."
However, an article published in the Journal of the American Medical Association seems to push back against Mallinckrodt's claims that the bad behavior was a "legacy" Questcor issue. The researchers who published their work in the journal investigated the way in which Acthar was prescribed in 2015, a year after Questcor merged with Mallinckrodt. They identified 300 doctors who prescribed Acthar ten or more times over the course of that year, resulting in more than $200 million billed to Medicare (about $667,000 per doctor). More than two thirds of those so-called "frequent prescribers" received payments from Mallinckrodt that in some cases were as high as six figures. In 2016, Mallinckrodt spent more than $8 million in payments to doctors related to Acthar, according to ProPublica.
"Not one thing has changed about Acthar since the 1950s. No improvement since it was invented, since someone took the fluid from the pituitary gland of a pig and injected it into a baby. All of a sudden the growth just ballooned in 2010, 2011. The only thing that's different is the marketing," Haviland said. "And the price."
A Litany of Litigation
Over the past several years, Mallinckrodt has found itself increasingly besieged by class action lawsuits, government scrutiny and activist investors hoping to make a killing in the company's demise.
In 2017, activist investor James Chanos referred to the relationship between Mallinckrodt and Express Scripts as a "murky alliance" and, along with other investment managers, began aggressively shorting the company. Chanos is best known for predicting and profiting from the collapse of Enron and, more recently, China's Luckin Coffee.
In August 2019, health insurance company Humana sued Mallinckrodt in federal court for $700 million, calling the company's handling of Acthar "one of the most outrageous price-gouging schemes in the history of American medicine."
A month later, Mallinckrodt paid $15.5 million to resolve the DOJ's and the whistleblowers' suit.
A board of education in Maryland also filed a lawsuit similar to Rockford's against Mallinckrodt and Express Scripts over the price its health plan paid for Acthar. That case ended in a judge's dismissal "on state law grounds." The current price for a vial of Acthar varies depending on who you ask. Mallinckrodt company literature from 2019 lists it at $38,892 a vial. Other third party sources put the cost at $40,613.
In addition to battling these Acthar-related suits, Mallinckrodt has spent the last several years fending off an enormous class-action lawsuit over their production of generic opioids. Oxycontin manufacturer Purdue Pharma has taken the brunt of the bad press for its role in the opioid epidemic, but according to data from the Washington Post, Mallinckrodt actually produced more pills than Purdue.
In February, Mallinckrodt reached a tentative deal to pay $1.6 billion to the thousands of state and local governments seeking damages resulting from the opioid crisis. Mallinckrodt's beleaguered stock price rose almost 40 percent following the announcement of the settlement.
Mallinckrodt and Express Scripts' full defenses against Rockford's allegations are still unknown. But as the parties head toward trial it is certain that this will be something of a David-versus-Goliath contest. The two companies post annual revenues in the billions. Rockford's total expenditures for 2019 amounted to less than $290 million.
The case is set to be in its discovery phase until at least Thanksgiving. Judge Johnston has acknowledged the disparity of resources between plaintiff and defendant, writing that "the main defendant is a large international pharmaceutical company with substantial resources" and saying in a pre-trial hearing, "The City of Rockford is barely keeping up with what they are supposed to be doing with keeping the lights on in the streets."
Ryan Krull is a freelance journalist and assistant teaching professor in the department of communication and media at University of Missouri-St. Louis.